Harvard Physicist Creates Metallic Hydrogen Using Diamond Vise
For some time, scientists have been fascinated by the concept of metallic hydrogen. Such an element is believed to exist naturally when hydrogen is placed under extreme pressures (like in the interior of gas giants like Jupiter). But as a synthetic material, it would have endless applications, since it is believed to have superconducting properties at room temperature and the ability to retain its solidity once it has been brought back to normal pressure.
For this reason, condensed matter physicists have been attempting to create metallic hydrogen for decades. And according to a recent study published in Science Magazine, a pair of physicists from the Lyman Laboratory of Physics at Harvard University claim to have done this very thing. If true, this accomplishment could usher in a new age of super materials and high-pressure physics.
The existence of metallic hydrogen was first predicted in 1935 Princeton physicists Eugene Wigner and Hillard Bell Huntington. For years, Isaac Silvera (the Thomas D. Cabot Professor at Harvard University) and Ranga Dias, a postdoctorate fellow, have sought to create it. They claim to have succeeded, using a process which they described in their recently-published study, “Observation of the Wigner-Huntington transition to metallic hydrogen“.

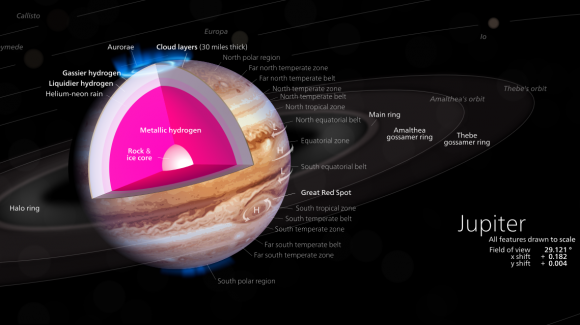
This cut-away illustrates a model of the interior of Jupiter, with a rocky core overlaid by a deep layer of liquid metallic hydrogen. Credit: Kelvinsong/Wikimedia Commons
Such a feat, which is tantamount to creating the heart of Jupiter between two diamonds, is unparalleled in the history of science. As Silvera described the accomplishment in a recent Harvard press release:
“This is the Holy Grail of high-pressure physics. It’s the first-ever sample of metallic hydrogen on Earth, so when you’re looking at it, you’re looking at something that’s never existed before.”
In the past, scientists have succeeded in creating liquid hydrogen at high temperature conditions by ramping up the pressures it was exposed to (as opposed to cryogenically cooling it). But metallic hydrogen has continued to elude experimental scientists, despite repeated (and unproven) claims in the past to have achieved synthesis. The reason for this is because such experiments are extremely temperamental.
For instance, the diamond anvil method (which Silvera and Dias used a variation of) consists of holding a sample of hydrogen in place with a thin metal gasket, then compressing it between two diamond-tipped vices. This puts the sample under extreme pressure, and a laser sensor is used to monitor for any changes. In the past, this has proved problematic since the pressure can cause the hydrogen to fill imperfections in the diamonds and crack them.
While protective coatings can ensure the diamonds don’t crack, the additional materials makes it harder to get accurate readings from laser measurements. What’s more, scientists attempting to experiment with hydrogen have found that pressures of ~400 gigapascals (GPa) or more need to be involved – which turns the hydrogen samples black, thus preventing the laser light from being able to penetrate it.
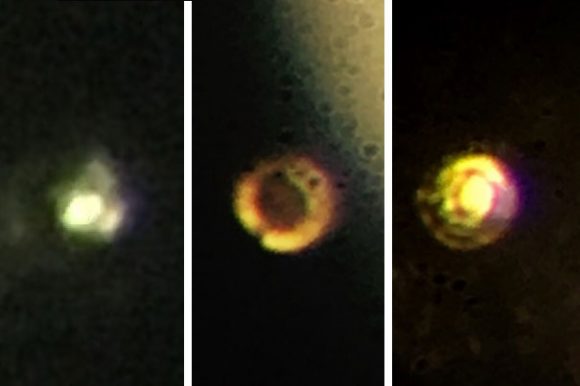
Microscopic images of the stages in the creation of metallic hydrogen: Transparent molecular hydrogen (left) at about 200 GPa, which is converted into black molecular hydrogen, and finally reflective atomic metallic hydrogen at 495 GPa. Credit: Isaac Silvera
For the sake of their experiment, Professors Ranga Dias and Isaac Silvera took a different approach. For starters, they used two small pieces of polished synthetic diamond rather than natural ones. They then used a reactive ion etching process to shave their surfaces, then coated them with a thin layer of alumina to prevent hydrogen from diffusing into the crystal structure.
They also simplified the experiment by removing the need for high-intensity laser monitoring, relying on Raman spectroscopy instead. When they reached a pressure of 495 GPa (greater than that at the center of the Earth), their sample reportedly became metallic and changed from black to shiny red. This was revealed by measuring the spectrum of the sample, which showed that it had become highly reflective (which is expected for a sample of metal).
As Silvera explained, these experimental results (if verified) could lead to all kinds of possibilities:
“One prediction that’s very important is metallic hydrogen is predicted to be meta-stable. That means if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure, but remain diamonds when that pressure and heat are removed. As much as 15 percent of energy is lost to dissipation during transmission, so if you could make wires from this material and use them in the electrical grid, it could change that story.”

Superconducting links developed to carry currents of up to 20,000 amperes are being tested at CERN. Credit: CERN
In short, metallic hydrogen could speed the revolution in electronics already underway, thanks to the discovery of materials like graphene. Since metallic hydrogen is also believed to be a superconductor at room temperature, its synthetic production would have immense implications for high-energy research and physics – such as that being conducted by CERN.
Beyond that, it would also enable research into the interior’s of gas giants. For some time, scientists have suspected that a layer of metallic hydrogen may surround the cores of gas giants like Jupiter and Saturn. Naturally, the temperature and pressure conditions in the interiors of these planets make direct study impossible. But by being able to produce metallic hydrogen synthetically, scientists could conduct experiment to see how it behaves.
Naturally, the news of this experiment and its results is being met with skepticism. For instance, critics wonder if the pressure reading of 495 GPa was in fact accurate, since Silvera and Dias only obtained that as a final measurement and were forced to rely on estimates prior to that. Second, there are those who question if the reddish speck that resulted is in fact hydrogen, and some material that came from the gasket or diamond coating during the process.
However, Silvera and Dias are confident in their results and believe they can be replicated (which would go far to silence doubts about their results). For one, they emphasize that a comparative measurement of the reflective properties of the hydrogen dot and the surrounding gasket suggest that the hydrogen is pure. They also claim their pressure measurements were properly calibrated and verified.
In the future, they intend to obtain additional spectrographic readings from the sample to confirm that it is in fact metallic. Once that is done, they plan to test the sample to see if it is truly metastable, which will consist of them opening the vise and seeing if it remains in a solid state. Given the implications of success, there are many who would like to see their experiment borne out!
Be sure to check out this video produced by Harvard University that talks about the experiment:
Further Reading: Science Magazine, Harvard Gazette
The post Harvard Physicist Creates Metallic Hydrogen Using Diamond Vise appeared first on Universe Today.
Universe Today
Go to Source
Powered by WPeMatico